Nós somos a Angelus

A Angelus é uma indústria de produtos odontológicos situada na cidade de Londrina, Paraná, que, desde a sua fundação em 1994, tem como missão “Gerar sorrisos saudáveis com inovação na Odontologia”. Para isso, investimos grande parte de nossos recursos na área de pesquisa e desenvolvimento e temos relacionamento estreito com Universidades e Centros de Pesquisa Tecnológicas para criar soluções inovadoras e que simplifiquem a rotina clínica dos dentistas.
Atualmente, nossos produtos atendem não apenas dentistas brasileiros como também profissionais de mais de 80 países nos 6 continentes.
Nossos resultados são decorrentes de inovações tecnológicas e investimentos, mas, acima de tudo, representam o empenho, o esforço e a dedicação de cada colaborador que trabalha na Angelus.
Acreditamos que o impossível é o que ainda não foi tentado!
Missão
Gerar sorrisos saudáveis com
inovação na Odontologia.
Visão
Ser reconhecidos globalmente como o melhor provedor de soluções inovadoras com foco na área de Endodontia.
Valores Angelus
Clique para conhecer em detalhes cada um de nossos valores!
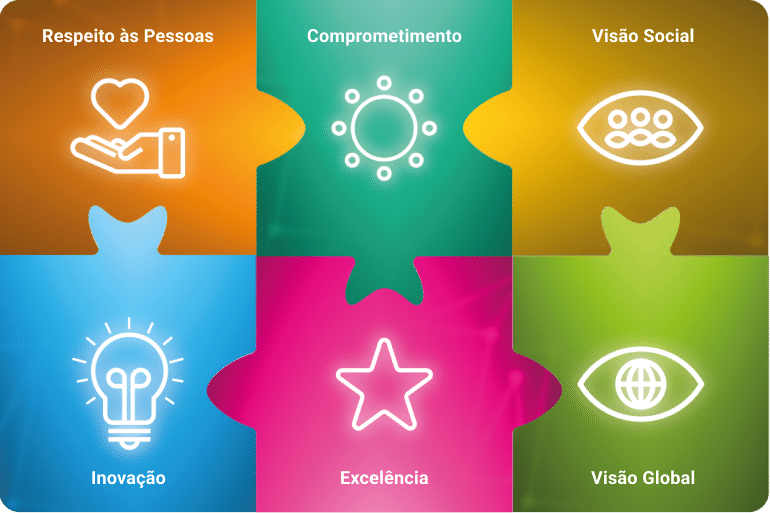


Visão Global
A atuação inovadora de forma consistente permite uma atuação global com geração de oportunidades para a sustentabilidade e perenidade do negócio.

Excelência
O comprometimento e a dedicação para fazer bem feito e de forma correta, na primeira vez, são os norteadores para atingir a qualidade e a mais alta satisfação do cliente.

Inovação
A inovação e a criatividade são responsáveis pelo nosso crescimento sustentável e para que sejamos reconhecidos mundialmente pela geração de soluções que facilitem os procedimentos e tragam resultados para o mercado.

Comprometimento
A proatividade e velocidade das entregas diante dos desafios, contribui de forma significativa para a eficiência dos processos com qualidade e integridade.

Respeito às pessoas
Respeito é essencial para as relações duradouras, e o nosso compromisso é direcionar todas as decisões a este valor, gerando, assim, um ambiente saudável entre os nossos colaboradores e todos os indivíduos ou grupos com os quais eles se relacionam.
Nossa Jornada
Conheça os principais marcos históricos ao longo de nossa trajetória:
Pessoas
Nos preocupamos com o bem-estar de nossos colaboradores, pois acreditamos que eles são os responsáveis por todos os resultados conquistados. Nosso ambiente de trabalho é ergonomicamente estruturado, com móveis e cadeiras projetados para atender a saúde e a segurança de todos. Acreditamos que um ambiente sadio e com clima de “segundo lar” faz com que as pessoas vistam a camisa da empresa e exponham todo o seu potencial criativo e inovador.

Estamos constantemente investindo em treinamento e desenvolvimento de nossos talentos para que estejam sempre atualizados com as melhores práticas de mercado, e este é um de nossos diferenciais: colaboradores talentosos, especializados e comprometidos!

Conquistamos pelo segundo ano consecutivo a certificação Great Place to Work (GPTW), por meio de uma pesquisa de Clima da GPTW. Esse selo nos enche de orgulho, pois comprova que estamos no caminho certo quando o assunto é o bem-estar das pessoas!

Prezamos pela constante valorização dos colaboradores que fazem parte do nosso time, pois, além de proporcionarmos um clima organizacional harmônico, também investimos em equipamentos de proteção individual e móveis ergonômicos, promovendo a conscientização sobre os cuidados com a saúde.
Depoimentos

PAS — Programa Angelus Socioambiental
A fim de espalhar ainda mais sorrisos, foi criado o Programa Angelus Socioambiental (PAS), que tem como objetivo desenvolver ações sustentáveis que contribuam com os Objetivos de Desenvolvimento Sustentável (ODS), estabelecidos pela Organização das Nações Unidas (ONU) em 2015. Conheça o programa de forma mais detalhada clicando no botão abaixo:
Portas abertas
Contamos com um programa regular de visitas à fábrica, no qual você poderá conhecer de perto o nosso processo industrial. Essa visita é indicada para a comunidade acadêmica, dentais, distribuidores, órgãos públicos e instituições de ensino.
Faça parte do nosso time!
Buscamos espalhar cada vez mais sorrisos saudáveis e desenvolver ainda mais o seu talento. Conheça as oportunidades de trabalho clicando no botão abaixo!
Angelus no mundo!
Desde a sua fundação, a Angelus se propõe a levar inovação e tecnologia para a Odontologia. Com essa estratégia conquistou mercados e atualmente exporta para mais de 80 países.